Pipeline
SOL-257 for Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease affecting approximately two to five of every 100,000 people worldwide. ALS is characterized by muscle weakness and paralysis as a result of motor neuron loss in the brain and spinal cord. Currently there is no cure for ALS. ALS patients are in urgent need of novel and effective treatments. Remarkably, most of ALS patients (>95%) have protein deposits in their degenerative neurons. These protein deposits always contain one protein called TDP-43. In the deposits, TDP-43 protein is abnormally folded (misfolding). Importantly, mutations in the gene encoding TDP-43 have also been found in some ALS patients. This evidence implies that TDP-43-targeting therapy to correct abnormally folded TDP-43 or to destroy the misfolded TDP-43 can be a promising therapy applicable to most ALS patients. This concept has been investigated in the last several years. However, the therapy targeting TDP-43 has not been realized yet primarily due to technical obstacles. The problem is that TDP-43 proteins are essential for our nervous system. Therefore, elimination of TDP-43 is not feasible. In the ALS patients, it is believed that there are two types of TDP-43. One is functional TDP-43 but the other is bad TDP-43 which is misfolded and thereby causes the disease. To develop an effective treatment targeting TDP-43, we need to discriminate good (functional) TDP-43 from bad TDP-43 (toxic misfolded TDP-43) and only bad TDP-43 needs to be eliminated, which is technically difficult.
Our designed JUMP70 technology targeting TDP-43 (SOL-257) enables specific elimination of “bad TDP-43”, and significantly improves protein folding issues associated with TDP-43 in cultured human cells. Experimental mouse expressing mutant TDP-43 develops TDP-43 protein folding issues in conjunction with neurotoxicity, leading to early death. We have further demonstrated that treatment of these mice with SOL-257 attenuated TDP-43 protein folding issues and significantly improved mouse survival.
SOL-175 for Huntington’s Disease (HD)
Huntington’s disease (HD) is a genetic neurodegenerative disorder that causes progressive chorea, dystonia, bradykinesia, motor incoordination, and cognitive decline. It affects over 30,000 Americans with more than 200,000 at-risk of inheriting the disease from a parent. HD pathogenesis is well defined; it is caused by expansion of a CAG trinucleotide repeat located in exon 1 of the Huntingtin gene, leading to an abnormal form of the Huntingtin protein (HTT) with polyQ tract (mutant HTT). The expanded polyQ stretch becomes abnormally folded and accumulates in oligomers and larger aggregates. This abnormally folded polyQ structure is likely to play a critical role in pathogenesis. Currently, HD cannot be prevented or cured; only symptomatic treatments are available.
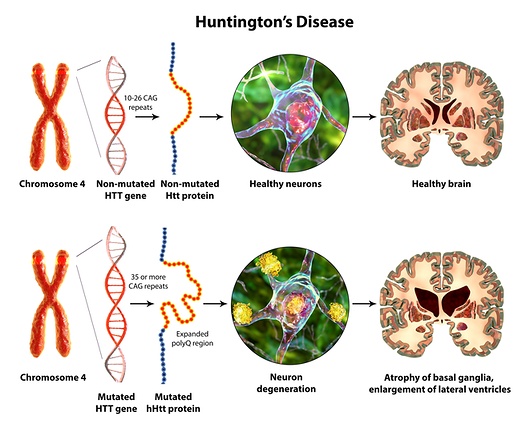
Our designed JUMP70 technology targeting polyQ stretch (SOL-175) can strikingly manipulate the protein folding process of mutant HTT proteins without affecting non-mutated HTT proteins, leading to our hypothesis that SOL-175 gene therapy can be a promising disease-modifying strategy for HD treatment.
© 2021 SOLA Biosciences